GxP Consultancy
GxP is a general term for Good x (anything) Practice quality guidelines and regulations.
Our expertise is in Good Clinical Practice (GCP), Good Laboratory Practice (GLP) and Good Manufacturing Practice in the Quality Control laboratory.
There's much more to a Quality System than just auditing!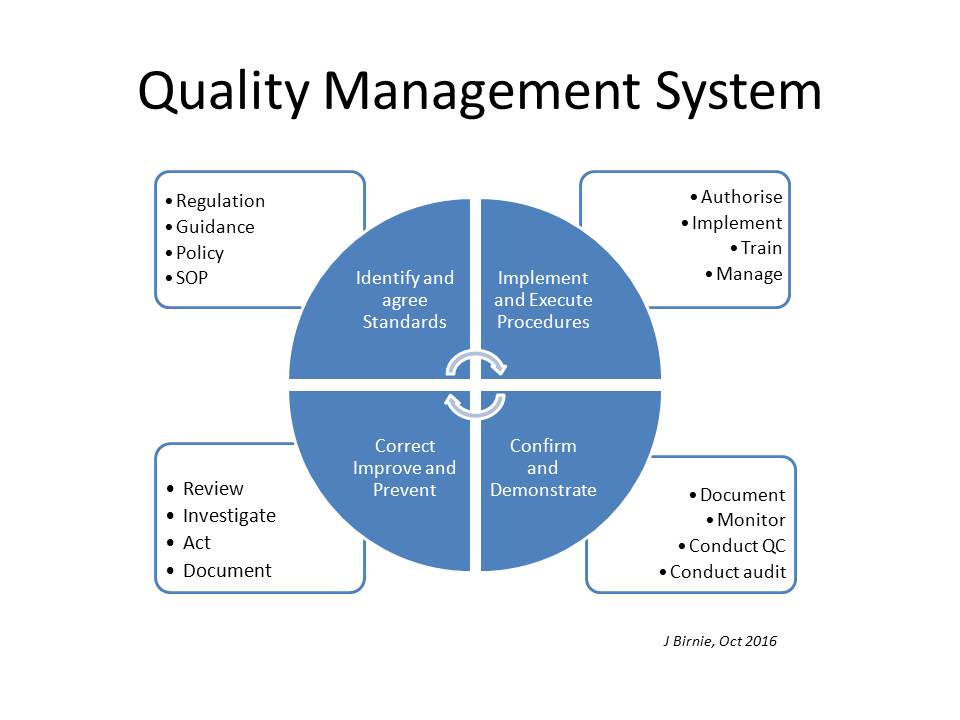
GCP Consultancy
• Regulatory Inspection preparation and follow-up:
◦Assessment of inspection-readiness of paper TMF, e-TMF or hybrid TMF
◦Inspection training “mock inspection”
◦Review and handling of inspection response
• Definition and demonstration of sponsor oversight activities
• Definition and implementation of data management QMS
Training
Training is provided in-house tailored to specific customer needs.
Some of our courses have been specifically for:
- Good Clinical Practice: introductory, refresher, updates
- Good Clinical Practice for Data Management
- Good Clinical Practice for Medical devices
- Good Clinical Practice for the Laboratory
- GCP and GLP in the Laboratory
- Good Manufacturing Practice for IMP
- Good Manufacturing Practice for Clinical professionals
GxP SOP Systems
- Diagnostic and Health check
- Re-engineering
Your QMS and SOPs should serve the business; not the other way around.
"Thank you again. Your preparation helped us a lot!"
Mock Inspection and GCP Regulatory Inspection Preparation,
Executive Clinical Director